Aztreonam Production Process with Cost Analysis: A Comprehensive Overview
Aztreonam, a monobactam antibiotic used primarily to treat bacterial infections, has become an essential pharmaceutical product in healthcare settings worldwide.
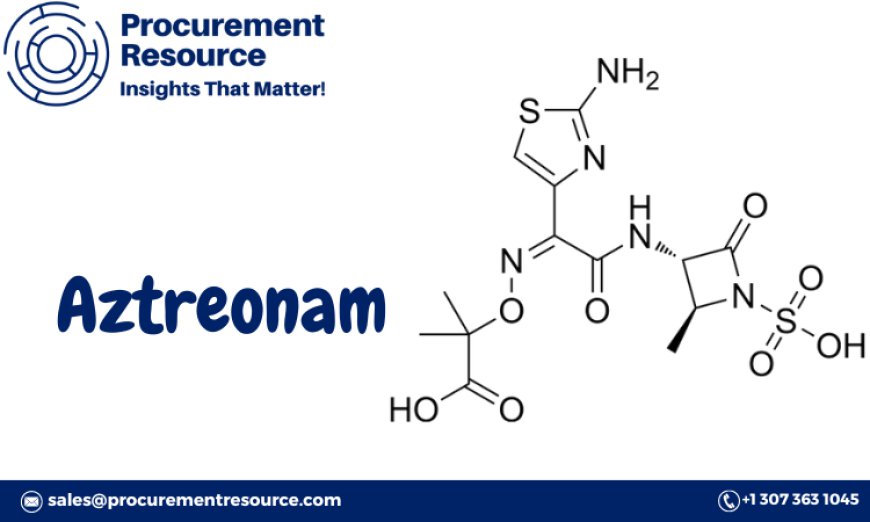
Aztreonam, a monobactam antibiotic used primarily to treat bacterial infections, has become an essential pharmaceutical product in healthcare settings worldwide. The Aztreonam Production Process with Cost Analysis offers valuable insights into the intricate process of manufacturing this life-saving drug. This report provides a detailed analysis of the procurement of key resources, an overview of Aztreonam, market drivers, raw material requirements, and a breakdown of costs associated with its production. Understanding the details of this process can significantly enhance the efficiency, sustainability, and cost-effectiveness of Aztreonam production.
Request For Free Sample: https://www.procurementresource.com/production-cost-report-store/aztreonam/request-sample
Procurement Resource Assessment for Aztreonam Production Process
Effective procurement resource assessment is a critical part of the Aztreonam production process. This involves evaluating the availability and quality of raw materials, suppliers, logistical efficiency, and overall supply chain management. Since Aztreonam is a highly specialized antibiotic, the procurement of resources must meet stringent quality and regulatory standards.
Key Considerations for Procurement Resource Assessment:
-
Quality of Raw Materials: For Aztreonam production, obtaining high-quality raw materials is critical. Any deviation in purity or quality can affect the efficacy and safety of the final product. Sourcing must focus on pharmaceutical-grade ingredients that meet international regulatory requirements.
-
Supplier Evaluation and Relationships: Establishing strong relationships with trusted suppliers ensures a consistent supply of high-quality raw materials. This also helps in negotiating favorable pricing, which can significantly impact production costs.
-
Logistics and Supply Chain Efficiency: Ensuring timely delivery of raw materials is essential for keeping the production process running smoothly. A robust supply chain with efficient transportation and warehousing facilities is crucial to avoid disruptions and ensure continuous production.
-
Regulatory Compliance: Procurement resources must comply with the strict regulatory standards imposed by global health authorities, including the FDA and EMA, to ensure that Aztreonam is manufactured in accordance with good manufacturing practices (GMP).
Effective procurement strategies enable manufacturers to minimize costs, maintain high production quality, and avoid disruptions in the supply chain.
What is Aztreonam?
Aztreonam is a synthetic monobactam antibiotic primarily used to treat Gram-negative bacterial infections, especially in patients with severe allergies to penicillin and other beta-lactam antibiotics. It is often administered intravenously or via inhalation for the treatment of pneumonia, urinary tract infections, skin infections, and other serious infections caused by susceptible bacteria.
Aztreonam’s mechanism of action involves inhibiting bacterial cell wall synthesis, thus preventing bacterial growth. One of the key benefits of Aztreonam is its ability to target specific Gram-negative bacteria, making it a critical antibiotic in hospital settings where resistant bacterial strains are a concern.
Due to the rise of antibiotic resistance and the increased demand for treatments that can combat these resistant strains, Aztreonam remains a vital component in the pharmaceutical arsenal against infections, further driving its production.
Market Drivers for Aztreonam Production
The demand for Aztreonam continues to grow due to several key market drivers, which have a direct impact on its production and market availability. Understanding these market drivers is crucial for manufacturers seeking to scale up production and maintain competitiveness.
Key Market Drivers:
-
Increasing Prevalence of Antibiotic-Resistant Infections: The global rise of antibiotic-resistant bacterial strains has led to an increased demand for antibiotics like Aztreonam, which is effective against certain resistant Gram-negative bacteria. Hospitals and healthcare providers rely on antibiotics like Aztreonam to combat infections that cannot be treated with conventional antibiotics.
-
Growing Demand in Healthcare Facilities: With rising global healthcare spending, hospitals, and healthcare providers are increasing their stock of critical antibiotics to address infections that require immediate intervention. This has expanded the market for Aztreonam, especially in critical care settings.
-
Advances in Drug Delivery Systems: Innovations in drug delivery methods, such as nebulized inhalation solutions for treating respiratory infections, have contributed to a surge in Aztreonam demand. These delivery systems enhance patient outcomes and allow for more effective treatment of specific infections.
-
Government Support and Initiatives: Several governments and international organizations are actively working to curb antibiotic resistance by funding research and development in novel antibiotics. Aztreonam, being a key player in the treatment of Gram-negative bacterial infections, benefits from these initiatives through regulatory approvals and increased production support.
-
Rising Incidences of Hospital-Acquired Infections (HAIs): The increasing number of HAIs, particularly in the ICU and post-operative settings, has spurred demand for reliable antibiotics like Aztreonam that are effective in treating these infections.
These market drivers underscore the growing importance of Aztreonam in the global healthcare system, making it essential for manufacturers to meet the rising demand by optimizing production processes.
Raw Material Requirements for Aztreonam Production
The production of Aztreonam involves several critical raw materials and intermediates that contribute to the synthesis and formulation of this antibiotic. Careful selection and procurement of these raw materials are crucial for maintaining product quality and ensuring compliance with pharmaceutical standards.
Key Raw Materials for Aztreonam Production:
-
Monobactam Core Structure: The synthesis of Aztreonam begins with the formation of its monobactam core, which is critical for its antibacterial properties. The synthesis of this core structure requires specialized chemical intermediates and reagents.
-
Chemical Intermediates: The production process involves a series of chemical reactions using specific intermediates that help in the formation of the active pharmaceutical ingredient (API). These intermediates must be of high purity to ensure the quality of the final product.
-
Solvents and Catalysts: Various solvents and catalysts are used during the synthesis and purification stages of Aztreonam production. The choice of solvent plays a crucial role in the efficiency of chemical reactions and the overall yield of the process.
-
Stabilizers and Additives: To ensure the stability and shelf-life of Aztreonam, certain stabilizers and additives are incorporated into the formulation. These are essential for maintaining the integrity of the product during storage and transportation.
-
Packaging Materials: Since Aztreonam is commonly delivered in injectable or inhalation form, high-quality packaging materials are needed to protect the product from contamination and ensure accurate dosing.
The selection and procurement of these raw materials are critical for maintaining consistent production quality, meeting regulatory standards, and minimizing production costs.
Costs and Key Process Information in Aztreonam Production
The Aztreonam production process involves several stages, each contributing to the overall production cost. A clear understanding of these key process steps and associated costs allows manufacturers to optimize their operations and reduce overheads.
Key Process Steps:
-
Chemical Synthesis: The production of Aztreonam starts with the chemical synthesis of the monobactam core. This step requires precise control of reaction conditions to achieve high yield and purity. The use of high-quality raw materials and catalysts is critical for this stage.
-
Purification: After synthesis, the crude Aztreonam undergoes purification to remove impurities and ensure that the final product meets the required pharmaceutical standards. This step involves filtration, crystallization, and other purification techniques.
-
Formulation: In the formulation stage, the purified Aztreonam is prepared into its final dosage form, which could be in the form of vials for injection or solutions for inhalation. This step may also involve the addition of stabilizers to enhance the product’s shelf life.
-
Quality Control: Rigorous quality control testing is conducted at various stages of production to ensure the product meets all regulatory standards. These tests include potency, purity, and stability assessments, which are crucial for ensuring the safety and efficacy of the final product.
-
Packaging and Distribution: Once the product passes all quality control tests, it is packaged into its final delivery system. Proper packaging is essential to protect the product from contamination and ensure proper dosing.
Cost Factors:
-
Raw Material Costs: The cost of raw materials, including the monobactam core, chemical intermediates, solvents, and stabilizers, is a major contributor to the overall production cost of Aztreonam.
-
Labor and Overhead: Skilled labor is required for the chemical synthesis, purification, and quality control stages. Additionally, facility overheads, such as equipment maintenance and energy consumption, contribute to production costs.
-
Regulatory Compliance: Pharmaceutical manufacturers must comply with strict regulatory standards, which involve significant costs related to inspections, certifications, and adherence to good manufacturing practices (GMP).
-
Research and Development: Ongoing R&D efforts to improve the production process, enhance product formulations, and develop new delivery systems also contribute to the overall cost of Aztreonam production.
By optimizing each stage of the production process and carefully managing costs, pharmaceutical companies can produce Aztreonam efficiently while maintaining a competitive edge in the market.
About Us:
Procurement Resource is an invaluable partner for businesses seeking comprehensive market research and strategic insights across a spectrum of industries. With a repository of over 500 chemicals, commodities, and utilities, updated regularly, they offer a cost-effective solution for diverse procurement needs. Their team of seasoned analysts conducts thorough research, delivering clients with up-to-date market reports, cost models, price analysis, and category insights.
By tracking prices and production costs across various goods and commodities, Procurement Resource ensures clients receive the latest and most reliable data. Collaborating with procurement teams across industries, they provide real-time facts and pioneering practices to streamline procurement processes and enable informed decision-making. Procurement Resource empowers clients to navigate complex supply chains, understand industry trends, and develop strategies for sustainable growth.
Contact Us:
Company Name: Procurement Resource
Contact Person: Benking sley
Email: sales@procurementresource.com
Toll Free Number: USA & Canada - Phone no: +1 307 363 1045 | UK - Phone no: +44 7537 132103 | Asia-Pacific (APAC) - Phone no: +91 1203185500
Address: 30 North Gould Street, Sheridan, WY 82801, USA
