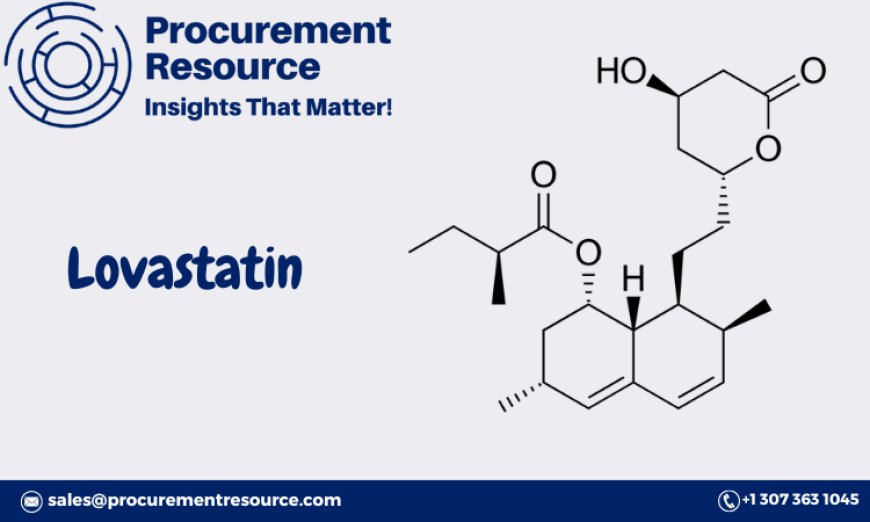
Lovastatin is a member of the statin family, widely used to lower cholesterol levels and reduce the risk of cardiovascular diseases. As an important pharmaceutical compound, understanding the production process of Lovastatin is crucial for optimizing cost, ensuring quality, and meeting the growing demand in the global market. This report provides a detailed analysis of the Lovastatin Production Process with Cost Analysis, covering procurement resource assessment, production steps, market drivers, raw materials, costs, and the benefits of a personalized report for businesses.
Request For Free Sample: https://www.procurementresource.com/production-cost-report-store/lovastatin/request-sample
Procurement Resource Assessment for Lovastatin Production Process
A vital aspect of Lovastatin production is the procurement of high-quality resources and materials that ensure both cost efficiency and adherence to regulatory standards. Several factors influence the procurement strategies for Lovastatin production:
1. Microbial Fermentation:
Lovastatin is predominantly produced through microbial fermentation using specific strains of the Aspergillus terreus fungus. This method relies heavily on the availability of biological cultures, specialized fermentation equipment, and skilled personnel who manage and optimize the process.
-
Culture Quality: Sourcing genetically stable and high-performing fungal strains is critical to achieving efficient production. Biotech companies specializing in microbial cultures provide Lovastatin producers with optimized strains.
-
Global Availability of Microbial Strains: While Aspergillus terreus is cultivated in controlled laboratory environments, global access to these microbial cultures ensures uninterrupted production cycles. Depending on the location of the production facility, logistics play a crucial role in procuring these cultures.
2. Fermentation Substrates:
Fermentation requires substrates like glucose or lactose, which serve as carbon sources for microbial growth. Selecting cost-effective and sustainable substrates is essential to minimizing operational expenses.
- Feedstock Pricing and Availability: The cost of substrates such as glucose or other carbon sources can fluctuate based on market conditions, geographical availability, and supplier networks. A robust procurement strategy involves multiple suppliers to ensure price stability and consistent supply.
3. Regulatory Compliance:
Procuring raw materials that comply with international quality and safety standards is vital in the pharmaceutical industry. Lovastatin producers must ensure that all raw materials, including microbial cultures and fermentation substrates, meet Good Manufacturing Practices (GMP) and are certified by regulatory authorities like the FDA (U.S.), EMA (Europe), and others.
Lovastatin: An Overview
Lovastatin is a naturally occurring statin, first isolated from Aspergillus terreus. It inhibits the enzyme HMG-CoA reductase, which is responsible for the synthesis of cholesterol in the liver. Lovastatin is used to lower cholesterol levels in patients with hypercholesterolemia and is also prescribed to reduce the risk of heart attacks, strokes, and other cardiovascular events.
Applications of Lovastatin:
-
Cholesterol Management: Lovastatin helps lower low-density lipoprotein (LDL) cholesterol, commonly known as "bad" cholesterol, while increasing high-density lipoprotein (HDL) cholesterol, the "good" cholesterol.
-
Prevention of Cardiovascular Diseases: By lowering cholesterol levels, Lovastatin reduces the risk of atherosclerosis, heart attacks, and strokes.
-
Other Uses: Emerging studies suggest that statins, including Lovastatin, may have potential benefits in other areas like cancer prevention and treatment, though these uses are still under investigation.
Market Drivers for Lovastatin Production
The global market for Lovastatin is influenced by several key drivers:
1. Increasing Incidence of Cardiovascular Diseases:
Cardiovascular diseases (CVDs) remain one of the leading causes of death worldwide. As sedentary lifestyles, poor dietary habits, and obesity contribute to the growing prevalence of hypercholesterolemia, the demand for statins, including Lovastatin, continues to rise.
- Aging Population: The aging global population is at an increased risk of cardiovascular diseases, leading to a higher demand for cholesterol-lowering medications like Lovastatin.
2. Regulatory Approvals and New Indications:
Lovastatin’s established safety profile has led to consistent approval from regulatory agencies across different countries. Additionally, ongoing research into the broader therapeutic applications of statins may open new markets and expand Lovastatin's usage.
- Patent Expirations: As Lovastatin’s patents have expired, generic formulations have flooded the market, increasing affordability and accessibility for patients globally.
3. Growing Awareness of Preventive Healthcare:
Patients are increasingly aware of the benefits of early diagnosis and preventive measures for cardiovascular diseases. This has led to greater use of cholesterol-lowering medications as part of routine health management, further driving demand.
4. Government Initiatives:
Several governments have initiated public health campaigns aimed at reducing cholesterol levels in their populations, which often involve subsidizing or promoting the use of statins like Lovastatin. These initiatives have boosted Lovastatin sales globally.
Raw Materials Requirements for Lovastatin Production
The production of Lovastatin through microbial fermentation involves several raw materials and process inputs:
1. Microbial Strains:
The core raw material for Lovastatin production is the Aspergillus terreus fungal strain, which produces Lovastatin naturally during fermentation. These strains are typically sourced from biotech firms specializing in the development of optimized microbial cultures.
- Quality and Performance: The selection of high-yielding fungal strains is essential for maximizing Lovastatin production. Strains must be genetically stable and capable of producing the drug at commercial scale.
2. Fermentation Substrates:
Lovastatin production requires substrates such as glucose, lactose, or other carbon sources that serve as nourishment for microbial growth during the fermentation process.
- Cost-effective Substrates: Depending on regional availability and cost, producers often choose substrates that are readily available at a competitive price. This helps to keep production costs low while ensuring high output.
3. Fermentation Media:
In addition to carbon sources, the fermentation media must include essential nutrients like nitrogen, phosphorus, and minerals to support the optimal growth of the Aspergillus terreus culture.
- Sustainable Sourcing: The sourcing of fermentation media ingredients must prioritize sustainability to ensure environmentally friendly production practices.
4. Utilities and Energy:
The fermentation process requires a stable supply of electricity, heat, and cooling to maintain optimal conditions for microbial growth. Energy costs can significantly impact production expenses, making energy-efficient systems an important aspect of cost management.
Costs and Key Process Information for Lovastatin Production
The cost of Lovastatin production can be broken down into several stages, each contributing to the overall manufacturing cost:
1. Fermentation:
The core process of Lovastatin production involves the fermentation of the Aspergillus terreus strain. This stage requires the highest input of raw materials and utilities.
-
Fermentation Equipment: Industrial fermentation tanks and bioreactors are required to grow the microbial cultures. These must be maintained under sterile conditions to avoid contamination, which adds to the capital and operational costs.
-
Process Monitoring: Continuous monitoring and control of pH, temperature, and oxygen levels are critical to optimizing the fermentation process. Automated control systems and skilled labor are necessary for process management, adding to operational costs.
2. Downstream Processing:
After fermentation, Lovastatin must be extracted and purified to meet pharmaceutical-grade standards. This involves several downstream processes, including filtration, centrifugation, and chromatography.
- Purification Costs: The cost of downstream processing depends on the level of purity required, the efficiency of extraction technologies, and the equipment used. Higher purity requirements often lead to increased costs.
3. Quality Control and Compliance:
Throughout the production process, Lovastatin must undergo rigorous quality control tests to ensure compliance with regulatory standards for pharmaceuticals. This involves testing for purity, potency, and the absence of contaminants.
- Regulatory Compliance Costs: Meeting GMP standards and adhering to the regulations set by agencies such as the FDA and EMA incurs costs related to testing, certification, and documentation.
4. Cost Breakdown:
- Raw Materials (Microbial Strains, Substrates): 50%-60% of total production cost
- Fermentation Equipment and Utilities: 15%-25%
- Downstream Processing and Purification: 10%-20%
- Labor and Quality Control: 5%-10%
By focusing on process optimization, reducing energy consumption, and ensuring sustainable sourcing of raw materials, Lovastatin producers can reduce overall production costs and improve profitability.
Looking for an Exhaustive and Personalized Report That Could Significantly Substantiate Your Business?
For companies engaged in the production or distribution of Lovastatin, having access to a detailed and personalized report can provide significant competitive advantages. Here’s how a comprehensive report can support your business:
-
Supply Chain Optimization: Gain insights into efficient procurement strategies for raw materials and explore new suppliers to reduce costs and enhance supply chain resilience.
-
Cost-saving Opportunities: Identify key cost drivers in the production process and discover areas where process optimization can lead to cost reductions.
-
Regulatory Compliance: Stay updated on the latest regulatory requirements and ensure your production process complies with international standards, avoiding costly fines and delays.
-
Market Insights: Access the latest market trends, competitor analysis, and demand forecasts to make informed decisions about expanding production capacity or entering new markets.
About Us: